 A couple of days ago one of the guys in the lab alerted my attention to a most interesting paper published in Organic Letters by Baldwin and co-workers from Oxford University. It's concerned with the postulated synthesis of a truncated analogue of Spiculoic Acid A by Mehta and Kundu. But before I get into this latest paper from Baldwin and his mates let's go back and see how it all started.
A couple of days ago one of the guys in the lab alerted my attention to a most interesting paper published in Organic Letters by Baldwin and co-workers from Oxford University. It's concerned with the postulated synthesis of a truncated analogue of Spiculoic Acid A by Mehta and Kundu. But before I get into this latest paper from Baldwin and his mates let's go back and see how it all started.It all took off in 2005 when Mehta and Kundu from the Indian Institute of Science in Bangalore published the following paper:
-
Toward a Total Synthesis of the Novel Polyketide Natural Product Spiculoic Acid A
Goverdhan Mehta and Uday Kumar Kundupp, Org. Lett., 2005, pp. 5569 - 5572
DOI: 10.1021/ol0521329
-
Now when I read this paper it actually came across as a nice piece of synthetic work. Unusually, these guys blatantly admit that their synthetic strategy towards the natural product failed. So what they do instead is provide a proof of concept by synthesising an analogue of the natural product using some Diels-Alder chemistry. Okay so that's all fine and dandy. At this point I'd like to say that I am very glad that I didn't referee this paper because things are about to get very hairy. Moving swiftly on to 2006 where Baldwin and co-workers publish the total synthesis of the enantiomer of Spiculonic Acid A in Chemical Communications:
-
Biomimetic synthesis of marine sponge metabolite spiculoic acid A and establishment of the absolute configuration of the natural product
James E. D. Kirkham, Victor Lee and Jack E. Baldwin, Chem. Commun., 2006, p. 2863
DOI: 10.1039/b607035c
DOI: 10.1039/b607035c
-
A very nice piece of synthetic work and a well written paper too. These dudes at Oxford really know what they are doing. So at this point I guess that Baldwin and his mates realised that there were some discrepancies between their data and those of Mehta and Kundu. Hence, they decided to sit down and dissect Mehta and Kundu's paper to figure out what was going on. The result of this little exercise was published in Organic Letters recently:
-
Stereochemical Reassignment of Mehta and Kundu's Spiculoic Acid A Analogue
Stereochemical Reassignment of Mehta and Kundu's Spiculoic Acid A Analogue
-
Now this paper is really worth a read. We are talking major bitch slapping here. To me the most unbelievable mistake is the incorrect stereochemical assignment of an epoxide obtained by a Sharpless asymmetric epoxidation. 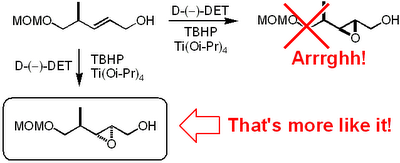 It appears that these Indian dudes haven't been able to use the mnemonic model published by Sharpless to predict the stereochemical outcome. This is what Mehta and Kundu write in their paper regarding the epoxidation:
It appears that these Indian dudes haven't been able to use the mnemonic model published by Sharpless to predict the stereochemical outcome. This is what Mehta and Kundu write in their paper regarding the epoxidation:
 It appears that these Indian dudes haven't been able to use the mnemonic model published by Sharpless to predict the stereochemical outcome. This is what Mehta and Kundu write in their paper regarding the epoxidation:
It appears that these Indian dudes haven't been able to use the mnemonic model published by Sharpless to predict the stereochemical outcome. This is what Mehta and Kundu write in their paper regarding the epoxidation:-
Sharpless epoxidation of allylic alcohol 19 in the presence of the D-tartaric acid diethyl ester was stereoselective (9:1) and afforded the epoxide 20 in a predictable manner with ample precedence.
-
And that's only the beginning. Their NOE interpretations are all over the place and it seems that they can't decide on the final stereochemistry of their Spiculonic Acid A when you compare the structures in the supplementary material with those given in the paper. Here's another brilliant quote from Mehta and Kundu's paper regarding their NOE interpretations (notice the language. One of these guys must have spent some time in the US and bought himself a dictionary):
-
The stereostructure of 9 was delineated on the basis of incisive analyses of its spectral characteristics, particularly the COSY and nOe data.
The stereostructure of 9 was delineated on the basis of incisive analyses of its spectral characteristics, particularly the COSY and nOe data.
-
Anyway, hats off to Baldwin and co-workers for spotting all the mistakes and submitting the paper and to Organic Letters for accepting it. It is quite remarkable to think that these guys from Oxford have managed to publish in Organic Letters without conducting a single experiment. I highly recommend reading these three papers in chronological order. D!
echo.....echo.....echo
ReplyDeleteI think i just saw a tumbleweed roll past.
Do you want to change the doi link to one that we all can access, not just those at Adelaide ;)
ReplyDeleteI think there is a mistake in your scheme. You would need to use L-(+)-DET to make the epoxide drawn in the box.
ReplyDeleteSorry PG... but you've made the same mistake as Mehta and Kundu! The (-) tartrate is correct.
ReplyDeleteDamn PG for a second I thought I couldn't use the menmonic either. Anyway, it seems that I did get it right after all. Thanks for keeping me on my toes. D!
ReplyDeleteD'oh! Apologies for the incorrect DOI's. It should be working now. Please let me know if it's still playing up. Cheers, D!
ReplyDeleteOh my God! What is the chem world coming to?? I think it's time the Queen of England give V. Lee a knighthood...it seems he's behind Sir Baldwin's highly prized corrections of others' claims!!
ReplyDelete