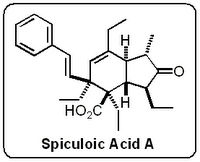
A couple of days ago one of the guys in the lab alerted my attention to a most interesting paper published in Organic Letters by Baldwin and co-workers from Oxford University. It's concerned with the postulated synthesis of a truncated analogue of
Spiculoic Acid A by
Mehta and
Kundu. But before I get into this latest paper from Baldwin and his mates let's go back and see how it all started.
It all took off in 2005 when
Mehta and
Kundu from the Indian Institute of Science in Bangalore published the following paper:
-
Toward a Total Synthesis of the Novel Polyketide Natural Product Spiculoic Acid A
Goverdhan Mehta and Uday Kumar Kundupp, Org. Lett., 2005, pp. 5569 - 5572
-
Now when I read this paper it actually came across as a nice piece of synthetic work. Unusually, these guys blatantly admit that their synthetic strategy towards the natural product failed. So what they do instead is provide a proof of concept by synthesising an analogue of the natural product using some Diels-Alder chemistry. Okay so that's all fine and dandy. At this point I'd like to say that I am very glad that I didn't referee this paper because things are about to get very hairy. Moving swiftly on to 2006 where Baldwin and co-workers publish the total synthesis of the enantiomer of Spiculonic Acid A in Chemical Communications:
-
Biomimetic synthesis of marine sponge metabolite spiculoic acid A and establishment of the absolute configuration of the natural product
James E. D.
Kirkham, Victor Lee and Jack E. Baldwin, Chem.
Commun., 2006, p. 2863
DOI:
10.1039/b607035c-
A very nice piece of synthetic work and a well written paper too. These dudes at Oxford really know what they are doing. So at this point I guess that Baldwin and his mates realised that there were some discrepancies between their data and those of Mehta and Kundu. Hence, they decided to sit down and dissect Mehta and Kundu's paper to figure out what was going on. The result of this little exercise was published in Organic Letters recently:
-
Stereochemical Reassignment of Mehta and Kundu's Spiculoic Acid A Analogue
Kirkham J. E. D., Lee, V. and Baldwin, J. E., Org.
Lett., 2006, ASAP Article
DOI:
10.1021/ol062361a -
Now this paper is really worth a read. We are talking major bitch slapping here. To me the most unbelievable mistake is the incorrect
stereochemical assignment of an
epoxide obtained by a
Sharpless asymmetric
epoxidation.
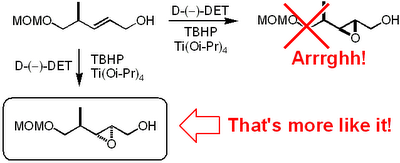
It appears that these Indian dudes haven't been able to use the mnemonic model published by
Sharpless to predict the
stereochemical outcome. This is what
Mehta and
Kundu write in their paper regarding the
epoxidation:
-
Sharpless epoxidation of allylic alcohol 19 in the presence of the D-tartaric acid diethyl ester was stereoselective (9:1) and afforded the epoxide 20 in a predictable manner with ample precedence.
-
And that's only the beginning. Their NOE interpretations are all over the place and it seems that they can't decide on the final stereochemistry of their Spiculonic Acid A when you compare the structures in the supplementary material with those given in the paper. Here's another brilliant quote from Mehta and Kundu's paper regarding their NOE interpretations (notice the language. One of these guys must have spent some time in the US and bought himself a dictionary):
-
The stereostructure of 9 was delineated on the basis of incisive analyses of its spectral characteristics, particularly the COSY and nOe data.
-
Anyway, hats off to Baldwin and co-workers for spotting all the mistakes and submitting the paper and to Organic Letters for accepting it. It is quite remarkable to think that these guys from Oxford have managed to publish in Organic Letters without conducting a single experiment. I highly recommend reading these three papers in chronological order. D!
 heteroaromatic systems that would make any pharma medchem person wet his pants. Moreover, they are doing it with excellent selectivity and in good yield. Most of the stuff in the paper is done under microwave conditions but they do one old school thermal example that works okay (See Scheme above).
heteroaromatic systems that would make any pharma medchem person wet his pants. Moreover, they are doing it with excellent selectivity and in good yield. Most of the stuff in the paper is done under microwave conditions but they do one old school thermal example that works okay (See Scheme above). However, the stuff in the JACS paper is different. Firstly, it only works if the carboxylic acid is adjacent to the heteroatom. Secondly, palladium adds adjacent to the carboxylic acid via an electrophilic palladation followed by palladium migration and concomitant decarboxylation. Finally the generated palladium species undergoes reductive elimination to form the desired product like this:
However, the stuff in the JACS paper is different. Firstly, it only works if the carboxylic acid is adjacent to the heteroatom. Secondly, palladium adds adjacent to the carboxylic acid via an electrophilic palladation followed by palladium migration and concomitant decarboxylation. Finally the generated palladium species undergoes reductive elimination to form the desired product like this:  In other words quite a different mechanism that doesn't involve a transmetallation step.
In other words quite a different mechanism that doesn't involve a transmetallation step.










 It appears that these Indian dudes haven't been able to use the mnemonic model published by
It appears that these Indian dudes haven't been able to use the mnemonic model published by  I've shown the reaction for an alkyne starting material but it also works for alkenes to give alcohols rather than ketones.
I've shown the reaction for an alkyne starting material but it also works for alkenes to give alcohols rather than ketones. Now these Scandos did a thorough job and wrote a fairly detailed experimental procedure:
Now these Scandos did a thorough job and wrote a fairly detailed experimental procedure:
 3) Removing "part of the acetic acid" should be changed to: "remove most of the acetic acid". Otherwise, you'll end up doing nothing but adding sodium bicarbonate and filtering bucket loads of sodium acetate off for an entire day (just like I did!).
3) Removing "part of the acetic acid" should be changed to: "remove most of the acetic acid". Otherwise, you'll end up doing nothing but adding sodium bicarbonate and filtering bucket loads of sodium acetate off for an entire day (just like I did!).  Now you have to admit that this oxidation uses the coolest reagents ever just judged by their abbreviations.
Now you have to admit that this oxidation uses the coolest reagents ever just judged by their abbreviations. 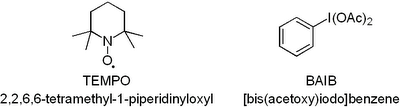 Both TEMPO and
Both TEMPO and  If you plan to hold on to it for a while it has to go in the freezer otherwise it goes off fast. Have fun, D!
If you plan to hold on to it for a while it has to go in the freezer otherwise it goes off fast. Have fun, D!


 orcid.org/0000-0003-3926-7047
orcid.org/0000-0003-3926-7047


