As I mentioned previously Professor Mukund Sibi also presented at the symposium. His talk was entitled: "
A New Dimension to Enantioselective Catalysis - Templates Come to the Rescue". Sibi is all about developing new methodologies for asymmetric synthesis. However, the approach is different from what other people in the area are doing. Basically, his concept is to attach a template to the molecule you would like to perform your asymmetric chemistry on. To achive asymmetric induction he now chucks some chiral Lewis acid into his flask followed by the reagent that is going to react with his substrate. The result is high yields and excellent ee's. Okay I think it's time for some structures to clarify matters. Sibi has done a whole bunch of asymmetric radical additions that goes along these lines:

A very important detail is that the template is a simple, achiral unit. The sole purpose of the template is to coordinate the Lewis acid well, exert rotamer control and as a consequence give good facial selective for the incoming nucleophile. Now as I mentioned before this principal works very well for many reactions. The Lewis acid is used in sub-stoichiometric quantities (generally 10-20 mol%). The radical stuff that I outlined above is okay cool but I personally like his stuff on pericyclic reactions better. Back in 2001 he published a very interesting paper in JACS (DOI:
10.1021/ja016396b) on Diels-Alder reactions:
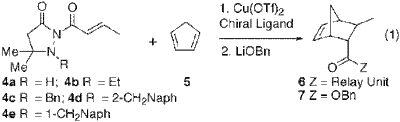
So this is taking things one step further by using a pyrazolidinone template with a substituted nitrogen. What they are achieving here is what can be described as relay induced enantioselectivity by nitrogen inversion. In other words, you use a achiral pyrazolidinone template and throw your chiral Lewis acid in that upon coordination will favour one asymmetric conformation of the template. Pretty funky stuff. You really need to check this paper out to get all the details. Anyway, it works very well. Here's some numbers:
Notice that template 11 with no relay unit is poor proving their point.
More recently Sibi has done some work on enantioselective [3+2] cycloaddition of nitrile imines (DOI:
10.1021/ja051650b). This time using their basic system with no relay. This stuff also works exceptionally well giving some heterocyclic compounds that might be appealing to people doing a bit of medicinal chemistry:

This time there's also regioselectivity issues. However, they solve this and all other associated problems elegantly producing the desired dihydropyrazoles in excellent yields and ee's.
I recommend reading these two JACS communications. Good thorough science and very well written papers. D!



 The Cambridge NMR service
The Cambridge NMR service  D!
D! A very important detail is that the template is a simple, achiral unit. The sole purpose of the template is to coordinate the Lewis acid well, exert rotamer control and as a consequence give good facial selective for the incoming nucleophile. Now as I mentioned before this principal works very well for many reactions. The Lewis acid is used in sub-stoichiometric quantities (generally 10-20 mol%). The radical stuff that I outlined above is okay cool but I personally like his stuff on pericyclic reactions better. Back in 2001 he published a very interesting paper in JACS (DOI:
A very important detail is that the template is a simple, achiral unit. The sole purpose of the template is to coordinate the Lewis acid well, exert rotamer control and as a consequence give good facial selective for the incoming nucleophile. Now as I mentioned before this principal works very well for many reactions. The Lewis acid is used in sub-stoichiometric quantities (generally 10-20 mol%). The radical stuff that I outlined above is okay cool but I personally like his stuff on pericyclic reactions better. Back in 2001 he published a very interesting paper in JACS (DOI: 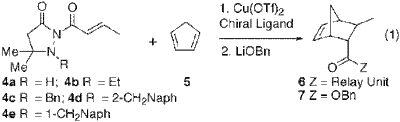

 This time there's also regioselectivity issues. However, they solve this and all other associated problems elegantly producing the desired dihydropyrazoles in excellent yields and ee's.
This time there's also regioselectivity issues. However, they solve this and all other associated problems elegantly producing the desired dihydropyrazoles in excellent yields and ee's.
 As Banwell pointed out the example with styrene is unbelievable and the ee's are through the roof! Moreover, the concept isn't limited to monosubstituted systems and more than 250 metabolites of this kind are known by now. So a couple of things immediately spring to mind. What scale can you do this sort of thing on and how do you get the other enantiomer of your product if that is what you are after. Well Banwell was on top of things and addressed these matters during his talk. Firstly, this stuff can be done on big scale. In the case of bromo- and chlorobenzene they obtain 35 grams of stuff per litre of fermentation broth which is pretty damn impressive. If you want the other enantiomer things are a bit trickier. However, Allen et al. have developed a method where the enantioselectivity is switched by introducing an iodine substituent that can be removed after the dihydroxylation:
As Banwell pointed out the example with styrene is unbelievable and the ee's are through the roof! Moreover, the concept isn't limited to monosubstituted systems and more than 250 metabolites of this kind are known by now. So a couple of things immediately spring to mind. What scale can you do this sort of thing on and how do you get the other enantiomer of your product if that is what you are after. Well Banwell was on top of things and addressed these matters during his talk. Firstly, this stuff can be done on big scale. In the case of bromo- and chlorobenzene they obtain 35 grams of stuff per litre of fermentation broth which is pretty damn impressive. If you want the other enantiomer things are a bit trickier. However, Allen et al. have developed a method where the enantioselectivity is switched by introducing an iodine substituent that can be removed after the dihydroxylation: A nice and simple solution to a complex problem that was published in Chemical Communications in 1995 (DOI:
A nice and simple solution to a complex problem that was published in Chemical Communications in 1995 (DOI: 

 orcid.org/0000-0003-3926-7047
orcid.org/0000-0003-3926-7047


