Friday, December 20, 2019
Bitopic Ligands and Epoxides
Tuesday, June 26, 2018
The Disconnection Approach - Automated!
 In my time as a synthetic organic chemist the most important advance in the field was definitely the introduction of searchable databases such as SciFinder and Reaxys. Life before these involved spending days on end in the library flipping though dusty tomes of chemical abstracts and Beilstein. And at the end you weren't even sure if you missed something of critical importance. The introduction of open access LC-MS and high field NMR has also had a big impact for me by speeding things up considerably. However, besides these milestones I think that I have pretty much been doing chemistry the same way >25 years. Anyway, what I am getting at is "what will the next BIG thing be?" It's been a considerable time since we had a major technical breakthrough for the synthetic organic chemist. My colleagues and I have been discussing this for more than a decade and now I think I am spotting the next big thing - Chematica! Chematica was developed by Polish scientist Grzybowski and has been around for quite a while. Basically it is a computer programme that disconnects your molecule and suggests ways for you to synthesise it. What's changed since I first heard mention of Chematica ~5 years ago is that apparently now it works the way you would like it to. Recently Grzybowski and co-workers published an impressive paper where they synthesise 8 very different and rather challenging molecules. The abstract from the paper nicely summarises the achievement:
In my time as a synthetic organic chemist the most important advance in the field was definitely the introduction of searchable databases such as SciFinder and Reaxys. Life before these involved spending days on end in the library flipping though dusty tomes of chemical abstracts and Beilstein. And at the end you weren't even sure if you missed something of critical importance. The introduction of open access LC-MS and high field NMR has also had a big impact for me by speeding things up considerably. However, besides these milestones I think that I have pretty much been doing chemistry the same way >25 years. Anyway, what I am getting at is "what will the next BIG thing be?" It's been a considerable time since we had a major technical breakthrough for the synthetic organic chemist. My colleagues and I have been discussing this for more than a decade and now I think I am spotting the next big thing - Chematica! Chematica was developed by Polish scientist Grzybowski and has been around for quite a while. Basically it is a computer programme that disconnects your molecule and suggests ways for you to synthesise it. What's changed since I first heard mention of Chematica ~5 years ago is that apparently now it works the way you would like it to. Recently Grzybowski and co-workers published an impressive paper where they synthesise 8 very different and rather challenging molecules. The abstract from the paper nicely summarises the achievement:Tuesday, October 06, 2009
How to Turn an Amine Into a Leaving Group
 gs generally go according to plan. However, what if you are stuck with an amine and want to substitute it with a nucleophile. There are a number of ways to do this but it's not just a walk in the park. Until recently I had never had to do this but then one fine morning I wanted to do the reaction above. How does one go about doing t
gs generally go according to plan. However, what if you are stuck with an amine and want to substitute it with a nucleophile. There are a number of ways to do this but it's not just a walk in the park. Until recently I had never had to do this but then one fine morning I wanted to do the reaction above. How does one go about doing t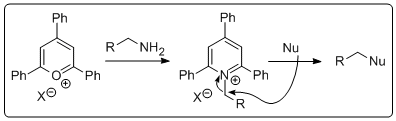 his? Is there a simple method by which I could activate the amine and displace it with the anion of 2-nitropropane, followed by a simple reduction to get the amine I wanted? Well, as it turns out Katritzky and co-workers published a paper in 1979 introducing triphenylpyrylium salts that can convert amines to leaving groups. Granted, the atom economy in this process is (to say th
his? Is there a simple method by which I could activate the amine and displace it with the anion of 2-nitropropane, followed by a simple reduction to get the amine I wanted? Well, as it turns out Katritzky and co-workers published a paper in 1979 introducing triphenylpyrylium salts that can convert amines to leaving groups. Granted, the atom economy in this process is (to say th e least) poor. However, the required pyrylium salt is commertcially available at a resonable price. All you do is stir it up with the amine. Prior to adding the amine the suspension is pale yellow and then when you toss the amine in it becomes a deep red slurry. In the photo the amine has just been added. It's always exciting with a bit of colour if your an organic chemist. The product is isolated by filtration. In this case the pyridinium salt was isolated as a light brown solid in 63% yield, perfectly clean by NMR. Next the pyridinium salt was treated with deprotonated 2-nitropropane in hot DMSO to give the nitro compound that was reduced using old school conditions. Interestingly, we could not get any reduction AT ALL of the nitro compound by catalytic hydrogentaion (at atmospheric pressure). Very odd! I would have ex
e least) poor. However, the required pyrylium salt is commertcially available at a resonable price. All you do is stir it up with the amine. Prior to adding the amine the suspension is pale yellow and then when you toss the amine in it becomes a deep red slurry. In the photo the amine has just been added. It's always exciting with a bit of colour if your an organic chemist. The product is isolated by filtration. In this case the pyridinium salt was isolated as a light brown solid in 63% yield, perfectly clean by NMR. Next the pyridinium salt was treated with deprotonated 2-nitropropane in hot DMSO to give the nitro compound that was reduced using old school conditions. Interestingly, we could not get any reduction AT ALL of the nitro compound by catalytic hydrogentaion (at atmospheric pressure). Very odd! I would have ex pected to see at least a few percent of the reduced stuff. Any ideas out there? Anyway, the amine was isolated in good yield over two steps after a short (2 cm tall) DCVC column. Yes a wastefull method but it is simple and fast. D!
pected to see at least a few percent of the reduced stuff. Any ideas out there? Anyway, the amine was isolated in good yield over two steps after a short (2 cm tall) DCVC column. Yes a wastefull method but it is simple and fast. D!Tuesday, January 13, 2009
The Skraup Reaction - How to Make a Quinoline


Tuesday, August 19, 2008
How to make a primary amide - The Ley Way
 So when Steven Ley comes up with a method that involves scooping solid magnesium nitride into a flask with your ester and some methanol, heating it to 80 oC for 24 hr, work up, filter, done! then that is really exciting good news to the synthetic organic chemist.
So when Steven Ley comes up with a method that involves scooping solid magnesium nitride into a flask with your ester and some methanol, heating it to 80 oC for 24 hr, work up, filter, done! then that is really exciting good news to the synthetic organic chemist.Monday, June 04, 2007
Asymmetric synthesis of vinylcyclopropanes
 The work is very throrough and makes up an 11 page JACS paper (not including any experimental). Many chemists would probably have split this work up in two papers. It's really nice to see these guys decided to stick the whole story in one paper. In brief these guys discover that they can make trisubstituted vinyl-cyclopropanes in high yield, diastereoselectivity and enantioselectivity. Moreover, they can make both enantiomers of cyclopropane selectively by switching from endo- to exo-sulfur ylides. This table from the paper illustrates how sweet this stuff is:
The work is very throrough and makes up an 11 page JACS paper (not including any experimental). Many chemists would probably have split this work up in two papers. It's really nice to see these guys decided to stick the whole story in one paper. In brief these guys discover that they can make trisubstituted vinyl-cyclopropanes in high yield, diastereoselectivity and enantioselectivity. Moreover, they can make both enantiomers of cyclopropane selectively by switching from endo- to exo-sulfur ylides. This table from the paper illustrates how sweet this stuff is: Only "problem" here is that they are using stoichiometric sulfur ylide. However, they address this by developing a catalytic ylide cyclopropanation. The yields are not as impressive and the ee's are down to 50-80%. Still pretty cool and I bet these guys are working hard to improve the catalytic system. Finally, they decide to pull off a short and high yielding formal total synthesis of a known cyclopropane amino acid.
Only "problem" here is that they are using stoichiometric sulfur ylide. However, they address this by developing a catalytic ylide cyclopropanation. The yields are not as impressive and the ee's are down to 50-80%. Still pretty cool and I bet these guys are working hard to improve the catalytic system. Finally, they decide to pull off a short and high yielding formal total synthesis of a known cyclopropane amino acid. Obviously, they are making both enantiomers as well as both enantiomers of a diastereoisomer. And here I'm messing around trying to improve my lousy dr's on the racemic synthesis of the same target. Crap! D!
Obviously, they are making both enantiomers as well as both enantiomers of a diastereoisomer. And here I'm messing around trying to improve my lousy dr's on the racemic synthesis of the same target. Crap! D!Tuesday, May 15, 2007
The Mannich Reaction revisited
 The Mannich Reaction (Carl Ulrich Franz Mannich, 1877-1947) is yet another one of those reactions that look brilliant on paper. However, I have on many occasions heard chemists attempting the reaction moan a fair bit to say the least. The major problem seems to be that the reaction is sluggish requiring heating/reflux to get anywhere and that the reagents (and desired product) start polymerising. You can find Mannich's original paper here: Mannich, C.; Krosche, W. Arch. Pharm. 1912, 250, p. 647. There is a detailed entry in Wikipedia on the reaction for those not familiar with it. A good alternative to the classic Mannich conditions is to use Eschenmoser's salt which I've seen used successfully in a number of total syntheses. Anyway, recently a PhD student in my lab was bitching about his Mannich Reaction. He left the lab, did some reading and came back with this nice JOC Note by A. Erkkila and P. M. Pihko, DOI: 10.1021/jo052529q. When he started using this stuff all his problems were solved. Fortunately, he sorted all this out right before I had to do my first Mannich Reaction. It also worked as a charm for me so I warmly recommend this simple, and efficient Mannich protocol.
The Mannich Reaction (Carl Ulrich Franz Mannich, 1877-1947) is yet another one of those reactions that look brilliant on paper. However, I have on many occasions heard chemists attempting the reaction moan a fair bit to say the least. The major problem seems to be that the reaction is sluggish requiring heating/reflux to get anywhere and that the reagents (and desired product) start polymerising. You can find Mannich's original paper here: Mannich, C.; Krosche, W. Arch. Pharm. 1912, 250, p. 647. There is a detailed entry in Wikipedia on the reaction for those not familiar with it. A good alternative to the classic Mannich conditions is to use Eschenmoser's salt which I've seen used successfully in a number of total syntheses. Anyway, recently a PhD student in my lab was bitching about his Mannich Reaction. He left the lab, did some reading and came back with this nice JOC Note by A. Erkkila and P. M. Pihko, DOI: 10.1021/jo052529q. When he started using this stuff all his problems were solved. Fortunately, he sorted all this out right before I had to do my first Mannich Reaction. It also worked as a charm for me so I warmly recommend this simple, and efficient Mannich protocol.
Now Erkkila and Pihko are quite concerned about reaction times because they are thinking of industry applications. However, for the average chemist that does a lot of work overnight (whilst at home in bed) it isn't essential that it's done in 1 hour. We found that if you do these reactions overnight no heating is required and the products are of very high purity. Very clean reactions indeed. Here's four examples from the paper:
 As it turns out the chemistry works really well for most systems using catalyst 1. However, some aldehydes require catalyst 2 to give a good result, eg. entries 3 and 4. The only compounds tested in this paper that failed completely were aldehydes that exist predominantly in a hemiacetal form, eg. 5-hydroxy-valeraldehyde. So there you have it. Maybe something you should consider giving a go next time it's alpha-methylenation time. D!
As it turns out the chemistry works really well for most systems using catalyst 1. However, some aldehydes require catalyst 2 to give a good result, eg. entries 3 and 4. The only compounds tested in this paper that failed completely were aldehydes that exist predominantly in a hemiacetal form, eg. 5-hydroxy-valeraldehyde. So there you have it. Maybe something you should consider giving a go next time it's alpha-methylenation time. D!
Friday, May 04, 2007
Tethered aminohydroxylations Donohoe stylie
(1) Donohoe et al., Chem Comm, 2001, pp 2078-2079 (DOI: 10.1039/b107253f)
TA of acyclic, allylic carbamates using tert-butyl hypochlorite as the reoxidant with 4 mol% osmium. Yields ranging from 41 to 61%. Here's a really nice example with a diene:

(2) Donohoe et al., JACS, 2002, pp 12934-12935 (DOI: 10.1021/ja0276117)
TA of cyclic, allylic carbamates using tert-butyl hypochlorite as the reoxidant with 4 mol% osmium. Yields ranging from 50 to 83%. Works for 6,7 and 8-membered rings but only 5-membered rings with exocyclic double bonds undergo aminohydroxylation. Here's another nice example making a protected amino-sugar:

(3) Donohoe et al., Org. Lett., 2004, pp 2583-2585 (DOI: 10.1021/ol049136i)
TA of chiral acyclic, allylic carbamates using tert-butyl hypochlorite as the reoxidant with 4 mol% osmium. Yields ranging from 57 to 74% with excellent syn-selectivity. Some very impressive examples of TA reactions in this paper, for example:

(4) Donohoe et al., JACS, 2006, pp 2514-2515 (DOI: 10.1021/ja057389g)
 Nice stuff innit and it gets better.
Nice stuff innit and it gets better.
So it took about 6 years to develop this methodology to the point where I believe it will start finding wide spread use in synthesis. I'm itching to try one of these for myself and I'm desperately looking for an excuse. If anyone has tried running some of these Donohoe TAs I would very much like to hear any comments - is it really as good as it looks on paper? D!
Friday, April 20, 2007
Tempo Oxidations Part II
 Fortunately, all the ingredients are reasonably affordable. Mixing it all up and adding the alcohol gives a biphasic reaction mixture that looks a bit like Schweppes Orange:
Fortunately, all the ingredients are reasonably affordable. Mixing it all up and adding the alcohol gives a biphasic reaction mixture that looks a bit like Schweppes Orange: However, unlike Delfourne et al. my final product wasn't clean after a simple work up. Succinimide was simply precipitating everywhere and hence some silica was required. In the end a filtration through a silica plug proved sufficient to give clean product on a reasonably large scale (18 grams) in excellent yield (97%). So despite the fact that a simple work up wasn't sufficient to clean the product up this is an easy to do reaction that I would recommend to anyone who's tired of stinky old Swern. D!
However, unlike Delfourne et al. my final product wasn't clean after a simple work up. Succinimide was simply precipitating everywhere and hence some silica was required. In the end a filtration through a silica plug proved sufficient to give clean product on a reasonably large scale (18 grams) in excellent yield (97%). So despite the fact that a simple work up wasn't sufficient to clean the product up this is an easy to do reaction that I would recommend to anyone who's tired of stinky old Swern. D! Monday, April 16, 2007
Fun with singlet oxygen
 Now the above reaction is obviously totally irresponsible and was only on for less than a minute so that I could take the picture. We are dealing with a fancy piece of glassware that has oxygen bubbling through it whilst two flood lamps are hammering photons away generating singlet oxygen and in the process heating the fume hood up big time. Hence, aluminium foil on the bottom of the hood is required to literally avoid a melt down and I'll be hooking a pump up that sends ice water through the cooling jacket. Moreover, I'm synthesising an endoperoxide - AAaARRRrGGGHhHh PEROXIDE - yeah I know but they are really quite stable. In fact our friends at the defence force haven't been able to blow them up so we aren't too worried. However, to avoid any nasty surprises we try not to make more than 5-10 grams of endoperoxides at any time. So as I said totally irresponsible hence I proceed to wrap this beautiful reaction up in two blasts shields covered in aluminum foil, pull the sash down and hook a cooling box/pump up to the glass ware and the final set up looks like this:
Now the above reaction is obviously totally irresponsible and was only on for less than a minute so that I could take the picture. We are dealing with a fancy piece of glassware that has oxygen bubbling through it whilst two flood lamps are hammering photons away generating singlet oxygen and in the process heating the fume hood up big time. Hence, aluminium foil on the bottom of the hood is required to literally avoid a melt down and I'll be hooking a pump up that sends ice water through the cooling jacket. Moreover, I'm synthesising an endoperoxide - AAaARRRrGGGHhHh PEROXIDE - yeah I know but they are really quite stable. In fact our friends at the defence force haven't been able to blow them up so we aren't too worried. However, to avoid any nasty surprises we try not to make more than 5-10 grams of endoperoxides at any time. So as I said totally irresponsible hence I proceed to wrap this beautiful reaction up in two blasts shields covered in aluminum foil, pull the sash down and hook a cooling box/pump up to the glass ware and the final set up looks like this: Well at least I know it's beautiful behind all that plastic and aluminium foil. This particular day I was doing the following photolysis:
Well at least I know it's beautiful behind all that plastic and aluminium foil. This particular day I was doing the following photolysis: 
Reactions of this type generally work quite well giving yields in the 40-70% range and since dienes are easily accessible using classic Wittig chemistry we consider making endoperoxides quite trivial. So why was I making this particular endoperoxide? Well if I told you I would have to kill you. There should be a paper coming out later this year featuring amongst others this particular endoperoxide turning into a supa cool cyclopropane in one (yes one) synthetic step so keep your eyes open for that paper. Some of you are probably wondering what Rose Bengal is. Behold the halogenated beast: We tend to use the bis-triethyl ammonium salt (as shown) because it is nicely soluble in organic solvents such as dichloromethane. D!
We tend to use the bis-triethyl ammonium salt (as shown) because it is nicely soluble in organic solvents such as dichloromethane. D!
Saturday, December 09, 2006
Adelaide Synthetic Symposium 2006 Part II
 A very important detail is that the template is a simple, achiral unit. The sole purpose of the template is to coordinate the Lewis acid well, exert rotamer control and as a consequence give good facial selective for the incoming nucleophile. Now as I mentioned before this principal works very well for many reactions. The Lewis acid is used in sub-stoichiometric quantities (generally 10-20 mol%). The radical stuff that I outlined above is okay cool but I personally like his stuff on pericyclic reactions better. Back in 2001 he published a very interesting paper in JACS (DOI: 10.1021/ja016396b) on Diels-Alder reactions:
A very important detail is that the template is a simple, achiral unit. The sole purpose of the template is to coordinate the Lewis acid well, exert rotamer control and as a consequence give good facial selective for the incoming nucleophile. Now as I mentioned before this principal works very well for many reactions. The Lewis acid is used in sub-stoichiometric quantities (generally 10-20 mol%). The radical stuff that I outlined above is okay cool but I personally like his stuff on pericyclic reactions better. Back in 2001 he published a very interesting paper in JACS (DOI: 10.1021/ja016396b) on Diels-Alder reactions:

 This time there's also regioselectivity issues. However, they solve this and all other associated problems elegantly producing the desired dihydropyrazoles in excellent yields and ee's.
This time there's also regioselectivity issues. However, they solve this and all other associated problems elegantly producing the desired dihydropyrazoles in excellent yields and ee's.Thursday, November 30, 2006
Pd-catalysed Cross-Coupling Reactions of Heteroaromatic Carboxylic Acids
-
 heteroaromatic systems that would make any pharma medchem person wet his pants. Moreover, they are doing it with excellent selectivity and in good yield. Most of the stuff in the paper is done under microwave conditions but they do one old school thermal example that works okay (See Scheme above).
heteroaromatic systems that would make any pharma medchem person wet his pants. Moreover, they are doing it with excellent selectivity and in good yield. Most of the stuff in the paper is done under microwave conditions but they do one old school thermal example that works okay (See Scheme above). However, the stuff in the JACS paper is different. Firstly, it only works if the carboxylic acid is adjacent to the heteroatom. Secondly, palladium adds adjacent to the carboxylic acid via an electrophilic palladation followed by palladium migration and concomitant decarboxylation. Finally the generated palladium species undergoes reductive elimination to form the desired product like this:
However, the stuff in the JACS paper is different. Firstly, it only works if the carboxylic acid is adjacent to the heteroatom. Secondly, palladium adds adjacent to the carboxylic acid via an electrophilic palladation followed by palladium migration and concomitant decarboxylation. Finally the generated palladium species undergoes reductive elimination to form the desired product like this:  In other words quite a different mechanism that doesn't involve a transmetallation step.
In other words quite a different mechanism that doesn't involve a transmetallation step.Anyway, thanks to aa for the tip. This has been most educational. D!
Thursday, November 09, 2006
How I learnt to love oxy-mercurations
 I've shown the reaction for an alkyne starting material but it also works for alkenes to give alcohols rather than ketones.
I've shown the reaction for an alkyne starting material but it also works for alkenes to give alcohols rather than ketones.
So why did I end up doing an oxy-mercuration? Well as it turns out we needed to get hold of a serious quantity of acetoxymethyl vinyl ketone and a quick search resulted in the following result: Now these Scandos did a thorough job and wrote a fairly detailed experimental procedure:
Now these Scandos did a thorough job and wrote a fairly detailed experimental procedure: A few small additions to the experimental procedure would be:
A few small additions to the experimental procedure would be:
1) During the acetylation the reaction is indeed somewhat exothermic and unintentionally I proceeded to boil the crap out of it (boils at ~150 oC) for about 5 minutes.
2) If reactions go jet black and shite starts precipitating from them this should be included in the experimental procedure. This is exactly what happens when you start adding the alkyne to the mercury cocktail. At this stage I was convinced that I had fried my alkyne but apparently black crap means that the reactions is working smoothly! This is what it looks like after the addition of a small amount of the alkyne: 3) Removing "part of the acetic acid" should be changed to: "remove most of the acetic acid". Otherwise, you'll end up doing nothing but adding sodium bicarbonate and filtering bucket loads of sodium acetate off for an entire day (just like I did!).
3) Removing "part of the acetic acid" should be changed to: "remove most of the acetic acid". Otherwise, you'll end up doing nothing but adding sodium bicarbonate and filtering bucket loads of sodium acetate off for an entire day (just like I did!).
Anyway, to finish this oxy-mercuration business the chemistry works very well but man I was working my ass off for two days to get it all done. There's quite a few time consuming steps in this prep such as the adjusting of the pH to 8 followed by removal of endless quantities of sodium acetate not to mention a vacuum distillation at the very end. D!





 orcid.org/0000-0003-3926-7047
orcid.org/0000-0003-3926-7047


