After this rather interesting paper on the oxidation powers of sodium hydride (That has been slapped around by the blogging community in a big way) it seems appropriate with a post on reagents that actually are capable of performing oxidations.
---
We all know pydridinium dichromate (PDC). It's one of these hopeless reagents that still gets taught on undergraduate chemistry courses despite the fact that A LOT has happened since 1979. I guess students should be aware of the existence of these reagents and maybe their use can be justified sometimes (Please let us know if you believe this to be the case). The "marvellous" thing about PDC is that it oxidises primary alcohols to aldehydes. And to be fair, when this was first discovered and described by Corey and Schmidt in 1979 it was probably an important contribution to synthetic organic chemistry (Click on image for enlargement).
 The fun part with PDC is making it which is very simple and produces a beautiful bright orange/metallic crystalline substance (See picture).
The fun part with PDC is making it which is very simple and produces a beautiful bright orange/metallic crystalline substance (See picture).
We all know pydridinium dichromate (PDC). It's one of these hopeless reagents that still gets taught on undergraduate chemistry courses despite the fact that A LOT has happened since 1979. I guess students should be aware of the existence of these reagents and maybe their use can be justified sometimes (Please let us know if you believe this to be the case). The "marvellous" thing about PDC is that it oxidises primary alcohols to aldehydes. And to be fair, when this was first discovered and described by Corey and Schmidt in 1979 it was probably an important contribution to synthetic organic chemistry (Click on image for enlargement).
 The fun part with PDC is making it which is very simple and produces a beautiful bright orange/metallic crystalline substance (See picture).
The fun part with PDC is making it which is very simple and produces a beautiful bright orange/metallic crystalline substance (See picture). However, this is where the fun stops. To oxidise a primary alcohol to an aldehyde we must expose it to stoichiometric (!!!) PDC. The reaction mixture is nasty (See picture of black suspension from hell).
However, this is where the fun stops. To oxidise a primary alcohol to an aldehyde we must expose it to stoichiometric (!!!) PDC. The reaction mixture is nasty (See picture of black suspension from hell). Finally when the reaction is finished you have to get rid of a lot of chromium stuff. Filtration through a tightly packed silica plug is the way forward. However, due to the presence of pyridine the chromium junk will start moving and co-eluting even in straight hexane (See picture of horrible filtration).
Finally when the reaction is finished you have to get rid of a lot of chromium stuff. Filtration through a tightly packed silica plug is the way forward. However, due to the presence of pyridine the chromium junk will start moving and co-eluting even in straight hexane (See picture of horrible filtration). There is a long, long list of old school reagents (e.g. Swern oxidation) and more modern ones (e.g. TPAP) that will carry this transformation out under much nicer conditions. TPAP (Tetra Propyl Ammonium Perruthanate) is a personal favourite that has worked wonders for me. TPAP is great for a number of reasons. Firstly, it is used in low catalyst loadings with the co-oxidant NMO (N-methylmorpholine N-oxide).
There is a long, long list of old school reagents (e.g. Swern oxidation) and more modern ones (e.g. TPAP) that will carry this transformation out under much nicer conditions. TPAP (Tetra Propyl Ammonium Perruthanate) is a personal favourite that has worked wonders for me. TPAP is great for a number of reasons. Firstly, it is used in low catalyst loadings with the co-oxidant NMO (N-methylmorpholine N-oxide). Secondly, the work-up is very simple normally just involving filtration through a plug of Celite followed by column chromatography. Some readers may have noticed that I on several occasions have mentioned some of Steven Ley's wonderful contributions to synthetic organic chemistry. Well TPAP is yet another of his little wonder reagents. The Ley group published a review on TPAP back in 1994 that illustrates its versatility. However, allow me to use one of my own examples where we compared PDC to TPAP. The oxidation of lactols to lactones can be tricky because of the equilibrium between open chain aldehyde and lactone, as illustrated.
Secondly, the work-up is very simple normally just involving filtration through a plug of Celite followed by column chromatography. Some readers may have noticed that I on several occasions have mentioned some of Steven Ley's wonderful contributions to synthetic organic chemistry. Well TPAP is yet another of his little wonder reagents. The Ley group published a review on TPAP back in 1994 that illustrates its versatility. However, allow me to use one of my own examples where we compared PDC to TPAP. The oxidation of lactols to lactones can be tricky because of the equilibrium between open chain aldehyde and lactone, as illustrated. However, both PDC and TPAP selectively oxidise to give the desired lactone. In this case PDC even when the rate enhancing additive pyridinium trifluoroacetate was added took 4 to 11 days to go to completion with 2 equivalents of oxidant. In the end high yields of clean material was obtained but as described above the work-up procedure is tedious. TPAP on the other hand provided the desired material overnight (In reality the reaction was probably done within an hour but I was at the pub at this point in time) followed by filtration and chromatography to give excellent yields of lactone. I should mention the major down side to TPAP. It is very expensive! However, due to low catalyst loadings, high yields, fast and simple purification I believe that the expense is easily justified for valuable starting materials. D!
However, both PDC and TPAP selectively oxidise to give the desired lactone. In this case PDC even when the rate enhancing additive pyridinium trifluoroacetate was added took 4 to 11 days to go to completion with 2 equivalents of oxidant. In the end high yields of clean material was obtained but as described above the work-up procedure is tedious. TPAP on the other hand provided the desired material overnight (In reality the reaction was probably done within an hour but I was at the pub at this point in time) followed by filtration and chromatography to give excellent yields of lactone. I should mention the major down side to TPAP. It is very expensive! However, due to low catalyst loadings, high yields, fast and simple purification I believe that the expense is easily justified for valuable starting materials. D! Fortunately, all the ingredients are reasonably affordable. Mixing it all up and adding the alcohol gives a biphasic reaction mixture that looks a bit like Schweppes Orange:
Fortunately, all the ingredients are reasonably affordable. Mixing it all up and adding the alcohol gives a biphasic reaction mixture that looks a bit like Schweppes Orange: However, unlike Delfourne et al. my final product wasn't clean after a simple work up. Succinimide was simply precipitating everywhere and hence some silica was required. In the end a filtration through a silica plug proved sufficient to give clean product on a reasonably large scale (18 grams) in excellent yield (97%). So despite the fact that a simple work up wasn't sufficient to clean the product up this is an easy to do reaction that I would recommend to anyone who's tired of stinky old Swern. D!
However, unlike Delfourne et al. my final product wasn't clean after a simple work up. Succinimide was simply precipitating everywhere and hence some silica was required. In the end a filtration through a silica plug proved sufficient to give clean product on a reasonably large scale (18 grams) in excellent yield (97%). So despite the fact that a simple work up wasn't sufficient to clean the product up this is an easy to do reaction that I would recommend to anyone who's tired of stinky old Swern. D!  Now you have to admit that this oxidation uses the coolest reagents ever just judged by their abbreviations.
Now you have to admit that this oxidation uses the coolest reagents ever just judged by their abbreviations. 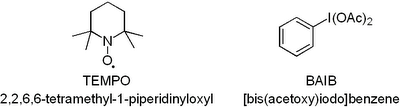 Both TEMPO and
Both TEMPO and  If you plan to hold on to it for a while it has to go in the freezer otherwise it goes off fast. Have fun, D!
If you plan to hold on to it for a while it has to go in the freezer otherwise it goes off fast. Have fun, D!


 orcid.org/0000-0003-3926-7047
orcid.org/0000-0003-3926-7047


