A while back Chris published a post on a Science paper entitled:
-
Synthesis of Biaryls via Catalytic Decarboxylative Coupling (DOI: 10.1126/science.1128684).
-
A very interesting piece of work. However, then one our readers known as aa posted the following comment:
-
"Not sure if this is referenced in the Science paper (should be), but similar work was recently reported by a group at Boehringer Ingelheim in JACS. Check out JACS, 2006, 128, 11350-11351. They use palladium catalysis, and heteroaryl carboxylic acids, but the principle is identical."
-
-
I finally did something about it and read the JACS paper (DOI:10.1021/ja063511f). Now first of all the German dudes who published their stuff in Science would have been hard pressed to cite the Boehringer Ingelheim group since their manuscript was submitted a month before the Boehringer people got round to it. The JACS paper is however very interesting. In fact, I find it even more exciting than the Science paper because these guys are making some pretty nice 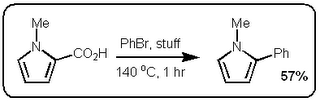 heteroaromatic systems that would make any pharma medchem person wet his pants. Moreover, they are doing it with excellent selectivity and in good yield. Most of the stuff in the paper is done under microwave conditions but they do one old school thermal example that works okay (See Scheme above).
heteroaromatic systems that would make any pharma medchem person wet his pants. Moreover, they are doing it with excellent selectivity and in good yield. Most of the stuff in the paper is done under microwave conditions but they do one old school thermal example that works okay (See Scheme above).
 heteroaromatic systems that would make any pharma medchem person wet his pants. Moreover, they are doing it with excellent selectivity and in good yield. Most of the stuff in the paper is done under microwave conditions but they do one old school thermal example that works okay (See Scheme above).
heteroaromatic systems that would make any pharma medchem person wet his pants. Moreover, they are doing it with excellent selectivity and in good yield. Most of the stuff in the paper is done under microwave conditions but they do one old school thermal example that works okay (See Scheme above).So at first glance these two papers seem very similar. However the postulated mechanisms are quite different. Firstly, the stuff in the Science paper starts of with a decarboxylation / copper insertion followed by a transmetallation and so forth. So in other words all the action is happening where the new bond is being made, like this:  However, the stuff in the JACS paper is different. Firstly, it only works if the carboxylic acid is adjacent to the heteroatom. Secondly, palladium adds adjacent to the carboxylic acid via an electrophilic palladation followed by palladium migration and concomitant decarboxylation. Finally the generated palladium species undergoes reductive elimination to form the desired product like this:
However, the stuff in the JACS paper is different. Firstly, it only works if the carboxylic acid is adjacent to the heteroatom. Secondly, palladium adds adjacent to the carboxylic acid via an electrophilic palladation followed by palladium migration and concomitant decarboxylation. Finally the generated palladium species undergoes reductive elimination to form the desired product like this:
 However, the stuff in the JACS paper is different. Firstly, it only works if the carboxylic acid is adjacent to the heteroatom. Secondly, palladium adds adjacent to the carboxylic acid via an electrophilic palladation followed by palladium migration and concomitant decarboxylation. Finally the generated palladium species undergoes reductive elimination to form the desired product like this:
However, the stuff in the JACS paper is different. Firstly, it only works if the carboxylic acid is adjacent to the heteroatom. Secondly, palladium adds adjacent to the carboxylic acid via an electrophilic palladation followed by palladium migration and concomitant decarboxylation. Finally the generated palladium species undergoes reductive elimination to form the desired product like this:  In other words quite a different mechanism that doesn't involve a transmetallation step.
In other words quite a different mechanism that doesn't involve a transmetallation step.Anyway, thanks to aa for the tip. This has been most educational. D!


 orcid.org/0000-0003-3926-7047
orcid.org/0000-0003-3926-7047



4 comments:
You are right, the pyrroles (indoles, furanes) are in a special category - first, their 2-carboxylic acids decarboxylate with great ease and second they easily metalate to 2-position with Pd(II). I think the benzoic acid example with Pd/Cu system is much more interesting mechanisticaly.
I think the one paper was deemed "Science-worthy" because it was used on a pilot plant scale. Both are great papers.
look at the cost of the catalyst in the JACS paper while the reagents are more simpler/cheaper in the Science. And if i remember right the reactions in the JACS paper required the 3 position to be susbtituted to get selectivity at 2-position.
Hi Guys!
I like your blog and I'm also particularly fond of catalysis and Pd chemistry. While I am in awe of what catalysis can do, my research is in inorganic chemistry. It's sorta cool not having to worry about Schlenk lines and drying solvents!
-Bkr
Post a Comment