Have you ever had to oxidise a primary alcohol to a carboxylic acid? Well as you know there is a ridiculous number of methods available. However, a common problem with many of the more traditional methods is that they are very harsh and could potentially rip your molecule apart. Just what you want after a 21-step linear synthesis innit? Moreover, many of the traditional metal-based oxidations can be a serious pain to work up so a mild and simple method would be kinda nice. Well one method that fulfills these criteria has been around for a while. I stumbled across it back in 1999 when I had to do one of these oxidations myself. A very nice piece of work on the oxidation of nucleosides:  Now you have to admit that this oxidation uses the coolest reagents ever just judged by their abbreviations. BAIB should obviously be pronounced BABE. Anyway, at first I had no idea what TEMPO and BAIB were:
Now you have to admit that this oxidation uses the coolest reagents ever just judged by their abbreviations. BAIB should obviously be pronounced BABE. Anyway, at first I had no idea what TEMPO and BAIB were: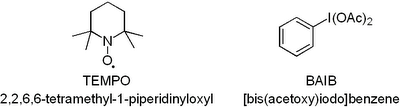 Both TEMPO and BAIB are commercially available. Don't you just love stuff like TEMPO. A radical you just scoop out of the flask and throw into your RBF! So how does the reaction work? We'll TEMPO does the hard work of oxidising the alcohol. However, as it is only used in a catalytic amount a stoichiometric amount of BAIB is required to regenerate TEMPO. As far as I know no one has yet figured the exact mechanism out. However, for those interested there is a good review in Synthesis that takes a close look at the mechanism and shows the most plausible pathways: Nooy et al., Synthesis, 1996, pp. 1153 - 1174. The paper is worth looking up just to check out the photos of the guys who wrote it - absolutely priceless! Anyway, to get to the point the method is very user-friendly you basically just mix a big pile of BAIB with a small quantity of TEMPO add your alcohol and some acetonitrile and water and stir it for a couple of hours. The method is compatible with a whole range of functional groups (double and triple bonds, esters, ethers, acetals, epoxides, amides, halides, and azides) as well as protection groups (TBDMS, THP, MOM, Boc, Cbz, Benzyl and acetyl etc.). I have recommended the method to a number of people and they have all used it with great success even with very sensitive compounds so I suggest you give it a go if you are in an oxidising mood.
Both TEMPO and BAIB are commercially available. Don't you just love stuff like TEMPO. A radical you just scoop out of the flask and throw into your RBF! So how does the reaction work? We'll TEMPO does the hard work of oxidising the alcohol. However, as it is only used in a catalytic amount a stoichiometric amount of BAIB is required to regenerate TEMPO. As far as I know no one has yet figured the exact mechanism out. However, for those interested there is a good review in Synthesis that takes a close look at the mechanism and shows the most plausible pathways: Nooy et al., Synthesis, 1996, pp. 1153 - 1174. The paper is worth looking up just to check out the photos of the guys who wrote it - absolutely priceless! Anyway, to get to the point the method is very user-friendly you basically just mix a big pile of BAIB with a small quantity of TEMPO add your alcohol and some acetonitrile and water and stir it for a couple of hours. The method is compatible with a whole range of functional groups (double and triple bonds, esters, ethers, acetals, epoxides, amides, halides, and azides) as well as protection groups (TBDMS, THP, MOM, Boc, Cbz, Benzyl and acetyl etc.). I have recommended the method to a number of people and they have all used it with great success even with very sensitive compounds so I suggest you give it a go if you are in an oxidising mood.
 Now you have to admit that this oxidation uses the coolest reagents ever just judged by their abbreviations. BAIB should obviously be pronounced BABE. Anyway, at first I had no idea what TEMPO and BAIB were:
Now you have to admit that this oxidation uses the coolest reagents ever just judged by their abbreviations. BAIB should obviously be pronounced BABE. Anyway, at first I had no idea what TEMPO and BAIB were: Both TEMPO and BAIB are commercially available. Don't you just love stuff like TEMPO. A radical you just scoop out of the flask and throw into your RBF! So how does the reaction work? We'll TEMPO does the hard work of oxidising the alcohol. However, as it is only used in a catalytic amount a stoichiometric amount of BAIB is required to regenerate TEMPO. As far as I know no one has yet figured the exact mechanism out. However, for those interested there is a good review in Synthesis that takes a close look at the mechanism and shows the most plausible pathways: Nooy et al., Synthesis, 1996, pp. 1153 - 1174. The paper is worth looking up just to check out the photos of the guys who wrote it - absolutely priceless! Anyway, to get to the point the method is very user-friendly you basically just mix a big pile of BAIB with a small quantity of TEMPO add your alcohol and some acetonitrile and water and stir it for a couple of hours. The method is compatible with a whole range of functional groups (double and triple bonds, esters, ethers, acetals, epoxides, amides, halides, and azides) as well as protection groups (TBDMS, THP, MOM, Boc, Cbz, Benzyl and acetyl etc.). I have recommended the method to a number of people and they have all used it with great success even with very sensitive compounds so I suggest you give it a go if you are in an oxidising mood.
Both TEMPO and BAIB are commercially available. Don't you just love stuff like TEMPO. A radical you just scoop out of the flask and throw into your RBF! So how does the reaction work? We'll TEMPO does the hard work of oxidising the alcohol. However, as it is only used in a catalytic amount a stoichiometric amount of BAIB is required to regenerate TEMPO. As far as I know no one has yet figured the exact mechanism out. However, for those interested there is a good review in Synthesis that takes a close look at the mechanism and shows the most plausible pathways: Nooy et al., Synthesis, 1996, pp. 1153 - 1174. The paper is worth looking up just to check out the photos of the guys who wrote it - absolutely priceless! Anyway, to get to the point the method is very user-friendly you basically just mix a big pile of BAIB with a small quantity of TEMPO add your alcohol and some acetonitrile and water and stir it for a couple of hours. The method is compatible with a whole range of functional groups (double and triple bonds, esters, ethers, acetals, epoxides, amides, halides, and azides) as well as protection groups (TBDMS, THP, MOM, Boc, Cbz, Benzyl and acetyl etc.). I have recommended the method to a number of people and they have all used it with great success even with very sensitive compounds so I suggest you give it a go if you are in an oxidising mood.And finally a practical note. If you like me have managed to get stuck in the middle of nowhere and hence has to wait for 9-12 months to receive your BAIB by ship from the US you may consider just making it yourself. I haven't tried this myself but the guys in the lab do it frequently using a simple prep from Synthesis: Kazmierczak et al., Synthesis, 1998, pp. 1721 - 1723. The final stuff is supposed to be bright yellow but the guys assure me that the pseudo-yellowish stuff you for unknown reasons obtain sometimes works just as well. Here's a picture of the pseudo-yellowish BAIB one of the guys made a couple of weeks ago:
 If you plan to hold on to it for a while it has to go in the freezer otherwise it goes off fast. Have fun, D!
If you plan to hold on to it for a while it has to go in the freezer otherwise it goes off fast. Have fun, D!


 orcid.org/0000-0003-3926-7047
orcid.org/0000-0003-3926-7047



22 comments:
I saw a TEMPO/BAIB oxidation that took a primary alcohol to the aldehyde. Any reason to think you can stop it there? These were process guys who were doing it with a substrate that was completely water soluable, hence the need for an oxidation system that required no aqueous workup.
Mayeb if you do this stuff anyhydrous it stops at the aldehyde. (oxidation of aldehyde to acid usually needs water - you actually oxidise the aldehyde hydrate)
Good question. The methodology is originally developed for the oxidation of primary and secondary alcohols to respectively aldehydes and ketones. The review I mention in the post is actually focused on this application: Nooy et al., Synthesis, 1996, pp. 1153 - 1174. The method is very mild and sometimes you can even selectively oxidise primary alcohols in the presence of secondary alcohols believe it or not. A common side product from these reactions is however carboxylic acids from overoxidation of the aldehyde hydrate. It was this fact that prompted some other guys to develop a method for taking the alcohol all the way to the carboxylic acid.
i Have always had good results with TEMPO oxidations, i highly recommend them. ( I am not sure about stopping at the CHO as mentioned above, that would be interesting.)
You can easily stop at the aldehyde in the two phase reaction. And you can use toluene as a solvent instead of DCM if emulsions are a problem.
Look at: Pier Lucio Anelli, Fernando Montanari, Silvio Quici Organic Syntheses Annual Volume 69, page 212
You can link directly to the reference from the TEMPO article in WikiPedia.
I'm just doing TEMPO oxidations and it's a brilliant reaction! I'm using bleach as co-oxidant (Tet Lett. 1992, 33, 5029) which should be accessible even in the outback!
Dear all, Could somebody tell me from which company I can buy BAIB best? I've looked at Sigma and Acros, but couldn't find it there.
Regards,
Dave
Dear aal,
Sorry for the last question, I found the product under diacetoxyiodobenzene and then it can be bought at Sigma or Acros.
However, I still have a question concerning removing the BAIB or its reduction product : how did anyone remove it from the reaction mixture?
Thanx,
Dave
Hi, Tha´nksfor your method introduction! but I have a big problem! BAIB is not soluble in CH3CN! didn#t you have any problem?
for transformation of alcohol to aldehyde, refer JOC, 1997, 62, 6974.
This paper shows many examples. maybe helpful.
Dave:
I found that the product of this oxidation (carboxylic acid) tends to precipitate out of solution as beads. It's beautiful, actually, perfectly spherical white beads in orange solution. These were nucleosides, though, I'm not sure what substrate you're working with.
Removal of BAIB and TEMPO is achieved by washing with ether and acetone.
Myriam:
The paper referenced in the post uses a 1:1 water:acetonitrile solvent mixture. You may need to add water to dissolve the BAIB (I've never fiddled with changing the procedure, so I'm not certain of this).
Is BAIB really bright yellow?
The stuff from Fluka (31490) is clear white powder. Following the paper Epp et al JOC1999, BAIB is not soluble in the amount of ACN:H2O described there. Moreover, addition of sodium bicarbonate formes voluminous precipitate and some gas bubbling. Has anybody experiences on this? Is the reaction mixture supposed to be heterogeneous or there is something wrong with my BAIB?
hi all
TEMPO-BAIB reaction is heterogeneous. Even BAIB is insouble in DCM, it works well. Advantage of this reaction, it is a mild oxidising agent. In some of the cases directly we convert the aldehyde to acid using Acetonitrile:Water system. Recently, many of the reserachers are utilizing this method
After I have tried everything to oxidize an alcohol group to acid, I decided to give this method a go, and it worked like a charm.
A little trick that I use to disolve the BAIB in the solvents mixture, is to sonicate the solution for a while. After that I add the TEMPO and wait for a couple of hours and it is done!
kaic: the BIAB should be a bright white solid. You can make it easily enough from sodium perborate, acetic acid, and iodobenzene. The yellow could be iodosylbenzene that is contaminating it.
I have just converted primary alcohol to Carboxylic acid. As a workup to confirm it, I am encountering two problems.
1. First, my product just dnt precipited out. So, I have to run a flash column chromatography.
2. Once, I got the pure product, I ran NMR. But Why I cant see peak for H of COOH in my NMR spectra.
I was wondering whether TEMPO/BAIB oxidation is selective enough for the oxidation of primary alcohol in the presence of secondary one, especially in the context of nucleic acids. If not, then will someone suggest me a suitable reagent for the selective oxidation. Any suggestions will be highly appreciated.
@Ajayachem
I just did the oxidation of a primary alcohol in the presence of both a secondary alcohol and a terminal double bond selectively towards the aldehyde using TEMPO/BAIB in DCM. Works like a charm.(J. Org. Chem. 1997, 62, 6974-6977)
Might this for some reason not work, you can also use trichloroisocyanuric acid and TEMPO. This did not work for me because of the double bond (I found polymerisation)but might work very well for you. (Organic letters, 2001, 3, 19, 3041-3043)
I recently did a TEMPO/BAIB oxidation of a furan based alcohol using DCM only as the solvent and reaction went smoothly to the aldehyde. Then used pinnick oxidation to get it to the acid.
Caution: A sample of BAIB we made in the lab exploded. May be room tempt (35 oC) and the sample being dry could be the problem. When wet it stays it stays OK.
Take DCM as solvent... Do Follow up the reaction. 1 h it will take....
Can this reaction be used for oxidizing aldehyde to carboxylic acid (i.e., not starting from alcohol, as it is described by Epp et al)?
Post a Comment