Leaving group activation of alcohols followed by nucleophilic substitution is routine stuff for the synthetic organic chemist. Just make the tosylate, nosylate, mesylate, triflate.... and thin gs generally go according to plan. However, what if you are stuck with an amine and want to substitute it with a nucleophile. There are a number of ways to do this but it's not just a walk in the park. Until recently I had never had to do this but then one fine morning I wanted to do the reaction above. How does one go about doing t
gs generally go according to plan. However, what if you are stuck with an amine and want to substitute it with a nucleophile. There are a number of ways to do this but it's not just a walk in the park. Until recently I had never had to do this but then one fine morning I wanted to do the reaction above. How does one go about doing t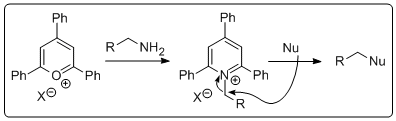 his? Is there a simple method by which I could activate the amine and displace it with the anion of 2-nitropropane, followed by a simple reduction to get the amine I wanted? Well, as it turns out Katritzky and co-workers published a paper in 1979 introducing triphenylpyrylium salts that can convert amines to leaving groups. Granted, the atom economy in this process is (to say th
his? Is there a simple method by which I could activate the amine and displace it with the anion of 2-nitropropane, followed by a simple reduction to get the amine I wanted? Well, as it turns out Katritzky and co-workers published a paper in 1979 introducing triphenylpyrylium salts that can convert amines to leaving groups. Granted, the atom economy in this process is (to say th e least) poor. However, the required pyrylium salt is commertcially available at a resonable price. All you do is stir it up with the amine. Prior to adding the amine the suspension is pale yellow and then when you toss the amine in it becomes a deep red slurry. In the photo the amine has just been added. It's always exciting with a bit of colour if your an organic chemist. The product is isolated by filtration. In this case the pyridinium salt was isolated as a light brown solid in 63% yield, perfectly clean by NMR. Next the pyridinium salt was treated with deprotonated 2-nitropropane in hot DMSO to give the nitro compound that was reduced using old school conditions. Interestingly, we could not get any reduction AT ALL of the nitro compound by catalytic hydrogentaion (at atmospheric pressure). Very odd! I would have ex
e least) poor. However, the required pyrylium salt is commertcially available at a resonable price. All you do is stir it up with the amine. Prior to adding the amine the suspension is pale yellow and then when you toss the amine in it becomes a deep red slurry. In the photo the amine has just been added. It's always exciting with a bit of colour if your an organic chemist. The product is isolated by filtration. In this case the pyridinium salt was isolated as a light brown solid in 63% yield, perfectly clean by NMR. Next the pyridinium salt was treated with deprotonated 2-nitropropane in hot DMSO to give the nitro compound that was reduced using old school conditions. Interestingly, we could not get any reduction AT ALL of the nitro compound by catalytic hydrogentaion (at atmospheric pressure). Very odd! I would have ex pected to see at least a few percent of the reduced stuff. Any ideas out there? Anyway, the amine was isolated in good yield over two steps after a short (2 cm tall) DCVC column. Yes a wastefull method but it is simple and fast. D!
pected to see at least a few percent of the reduced stuff. Any ideas out there? Anyway, the amine was isolated in good yield over two steps after a short (2 cm tall) DCVC column. Yes a wastefull method but it is simple and fast. D!
 gs generally go according to plan. However, what if you are stuck with an amine and want to substitute it with a nucleophile. There are a number of ways to do this but it's not just a walk in the park. Until recently I had never had to do this but then one fine morning I wanted to do the reaction above. How does one go about doing t
gs generally go according to plan. However, what if you are stuck with an amine and want to substitute it with a nucleophile. There are a number of ways to do this but it's not just a walk in the park. Until recently I had never had to do this but then one fine morning I wanted to do the reaction above. How does one go about doing t his? Is there a simple method by which I could activate the amine and displace it with the anion of 2-nitropropane, followed by a simple reduction to get the amine I wanted? Well, as it turns out Katritzky and co-workers published a paper in 1979 introducing triphenylpyrylium salts that can convert amines to leaving groups. Granted, the atom economy in this process is (to say th
his? Is there a simple method by which I could activate the amine and displace it with the anion of 2-nitropropane, followed by a simple reduction to get the amine I wanted? Well, as it turns out Katritzky and co-workers published a paper in 1979 introducing triphenylpyrylium salts that can convert amines to leaving groups. Granted, the atom economy in this process is (to say th e least) poor. However, the required pyrylium salt is commertcially available at a resonable price. All you do is stir it up with the amine. Prior to adding the amine the suspension is pale yellow and then when you toss the amine in it becomes a deep red slurry. In the photo the amine has just been added. It's always exciting with a bit of colour if your an organic chemist. The product is isolated by filtration. In this case the pyridinium salt was isolated as a light brown solid in 63% yield, perfectly clean by NMR. Next the pyridinium salt was treated with deprotonated 2-nitropropane in hot DMSO to give the nitro compound that was reduced using old school conditions. Interestingly, we could not get any reduction AT ALL of the nitro compound by catalytic hydrogentaion (at atmospheric pressure). Very odd! I would have ex
e least) poor. However, the required pyrylium salt is commertcially available at a resonable price. All you do is stir it up with the amine. Prior to adding the amine the suspension is pale yellow and then when you toss the amine in it becomes a deep red slurry. In the photo the amine has just been added. It's always exciting with a bit of colour if your an organic chemist. The product is isolated by filtration. In this case the pyridinium salt was isolated as a light brown solid in 63% yield, perfectly clean by NMR. Next the pyridinium salt was treated with deprotonated 2-nitropropane in hot DMSO to give the nitro compound that was reduced using old school conditions. Interestingly, we could not get any reduction AT ALL of the nitro compound by catalytic hydrogentaion (at atmospheric pressure). Very odd! I would have ex pected to see at least a few percent of the reduced stuff. Any ideas out there? Anyway, the amine was isolated in good yield over two steps after a short (2 cm tall) DCVC column. Yes a wastefull method but it is simple and fast. D!
pected to see at least a few percent of the reduced stuff. Any ideas out there? Anyway, the amine was isolated in good yield over two steps after a short (2 cm tall) DCVC column. Yes a wastefull method but it is simple and fast. D!

 orcid.org/0000-0003-3926-7047
orcid.org/0000-0003-3926-7047



16 comments:
I think you ought to try Pt-C for nitro reduction in EtOAc or THF under baloon. Pt is far more active than Pd for nitros, and I never had problems with ring reduction under baloon. But LAH should work also (hat tip to Dr. Shulgin).
2-Nitropropane alkylation is not a trivial stuff. Infact there is a method for transforming hindered benzylic chlorides (and bromides) to corresponding aldehydes, by treating them with sodium salt of 2-nitropropane. In this way 2,4-bis-chloromethyl mesytylene can be turned into the corresponding dialdehyde of trimethyl isophtalic acid, a precursor for important bis-nitriloxide crosslinker (in rubber industry) that is hard to access by other methods
Nice colors! I'll keep the reaction in mind, though I don't do much synthesis these days. :(
I am a BIG fan of Zn/AcOH for reducing ArNO2 groups. You might also try Fe. Interest note, I was told the first example of this called for 3 rusty nails. Hope it works.
b
did you try in situ reduction with ammonium formate and Pd/C at reflux? that's what i would prefer because removing the zinc stuff is sometimes very nasty...
Also had to go "old school" for a very similar nitro reduction. Found Sn/HCl worked very well but that isolation of the product (extraction after neutralization) was difficult resulting in low yields. Eventually got around this by using Sn/HCl in 20% isopropanol. Apart from solubilising the nitro compound on cooling the HCl salt of the amine crystallized out of the reaction mixture!
I've started using indium a lot for nitro reductions, very clean
I didn't have any trouble with the work-up of my Zn reduction (run in EtOH with 4M HCl) and I used approx. 6 g of Zn. I cooled my reaction mixture adjusted the pH to approx. 8 by adding sat. aq. NaHCO3, let it warm to RT and did a gravimetric filtration. The EtOH was removed in vacuo and the residual solvent extracted with EtOAc, followed by standard washing, drying etc. D!
Enjoy the posts! Keep updating please!!
Interesting post! Haven´t seen this reaction before.
Milkshake's second comment is about the Hass-Bender Oxidation. Reacts via O alklyation and gives a Swern-like intermediate and elimnination (of acetone oxime). Works quite nicely with other substrates if you can avoid (to much) C-alklyation.
can I do first step in water??
First post, but ammonium formate and Pd-C is my standard procedure. The NH4O2CH decomposes to H2 but effectively gives high conc. of H2 in situ w/o high pressure of say a par shaker. Anyways, hope it helps
can you blast 2-naphthylacetonitrile with CH3MgBr to get to your target?
Unfortunately nitriles with Grignards tend to stop only at imines - although I saw recently a procedure with methyl cerium that gave this kind of tertiary amine product (but it was not pretty - have you ever tried to make/use anhydrous CeCl3?). Plus, with arylacetonitrile you would get problems with enolyzation, that CH2 is awfully acidic.
I think a suitable alternate route would be based of Curtius or Hofmann degradation but it is a bit more steppy
Hello. I like the reaction very much. One other route which comes to mind is the Ritter on the corresponding tertiary alcohol followed by deprotection, although the alcohol itself may be difficult to source.
As for reductions on nitros, my personal favourite is Fe in acetic, although sodium dithionite is also a useful means.
I've never heard of that reaction before.
Where can I find the mechanism for the conversion of the pyrylium salt to the pyridinium salt?
Post a Comment