Synthesis of Biaryls via Catalytic Decarboxylative Coupling
Lukas J. Gooßen, Guojun Deng, and Laura M. Levy
Science 4 August 2006: Vol. 313. no. 5787, pp. 662 - 664
DOI: 10.1126/science.1128684
The fancy thing is that they generate the carbon nucleophile in situ by a copper-catalyzed decarboxylation of an arylcarboxylic acid salt. Hence, no lithiation and no handling of organometallic reagents.
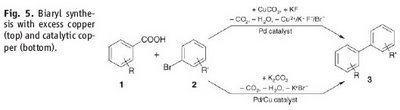
So far they have only been able to get the catalytic system (Cu/Pd) working for aryls with coordinating ortho-substituents, while stoichiometric amounts of copper are necessary for other systems. But still pretty sweet.


 orcid.org/0000-0003-3926-7047
orcid.org/0000-0003-3926-7047



4 comments:
Pretty nice method. Although, there are still some problems, such as high temperature and long reaction time, which for "large scale procedure" were 160C and overnight. However, only 0.5 mole per cent of Pd-catalyst was needed which is good.
Anyway, it's always nice to see synthetic organic chemistry in one of those top journals, i.e. Science or Nature.
Good one! I never bother checking Science and Nature myself since there hardly ever is any synthetic organic chemistry being published. This is a very nice piece of work indeed. Really low catalyst loading and high yields.
Last year I was entertaining a Professor in organic chemistry from the UK for a day. He'd recently published a paper in Nature and he thought that one of the reasons we hardly ever see any synthetic organic papers in these top journals is that people don't even consider submitting it to them. I think he has a good point. Personally, I regularly see stuff in JACS and Angewandte where I think that could possibly have made it into Nature or Science. D!
Not sure if this is referenced in the Science paper (should be), but similar work was recently reported by a group at Boehringer Ingelheim in JACS. Check out JACS, 2006, 128, 11350-11351. They use palladium catalysis, and heteroaryl carboxylic acids, but the principle is identical.
hey curly arrow!! i came across this thread while looking through google for aryl aryl coupling. im an MSc student in mumbai lookin to do a similar reaction. where can i get this whole paper? do u have it? may i get a soft copy at risad_18@hotmail.com. any help mwould be highly appreciated.
Post a Comment