 Old school 1,4-butynediol. A wondeful and cheap building block. D!
Old school 1,4-butynediol. A wondeful and cheap building block. D!
Wednesday, October 21, 2009
Sunday, October 18, 2009
Curly Arrow - Established 18th October 2006
 Three years later. I'm surprised that I still manage to keep Curly Arrow alive. The reason that things are still happening is you guys that read my stuff and send me some very positive and enthusiastic emails. I'm getting a lot of hits from Google and the blog appears to have a tremendous impact. For example if you do a Google search on Click Chemistry you'll see the Wikipedia entry as the top hit followed by Curly Arrow. Curly Arrow has a higher rating than the original literature on Click Chemistry! So I guess I'll just keep at it and see what happens. I have listed the stats for the past year below. Numbers are up but the top 10 visitors (that I can identify) haven't changed much. The major difference is that the only company (GSK) is out and that Japan has made an entry. I can see a whole bunch of european networks that I get a ton of hits from and I suspect that certain German Universities should be on the list. As a consequence this year I have also included top 10 contries that visit the blog. D!
Three years later. I'm surprised that I still manage to keep Curly Arrow alive. The reason that things are still happening is you guys that read my stuff and send me some very positive and enthusiastic emails. I'm getting a lot of hits from Google and the blog appears to have a tremendous impact. For example if you do a Google search on Click Chemistry you'll see the Wikipedia entry as the top hit followed by Curly Arrow. Curly Arrow has a higher rating than the original literature on Click Chemistry! So I guess I'll just keep at it and see what happens. I have listed the stats for the past year below. Numbers are up but the top 10 visitors (that I can identify) haven't changed much. The major difference is that the only company (GSK) is out and that Japan has made an entry. I can see a whole bunch of european networks that I get a ton of hits from and I suspect that certain German Universities should be on the list. As a consequence this year I have also included top 10 contries that visit the blog. D!-
Absolute unique visitors: 28,799 (previous year 21,250)
Total visits: 50,230 (138 Visits/Day) [previous year 37,513 (103 Visits/Day)]
Average time on site: 1:45 minute (previous year 1:28 minute)
The 10 most frequent visitors identifiable:
(1) Princeton University (last year: Princeton University)
(2) Scripps Research Institute (last year: Scripps Research Institute)
(3) Oxford University (last year: Oxford University)
(4) Ohio State University (last year: University of Cambridge)
(5) Kyoto University (last year: Flinders University)
(6) State University of New York at Buffalo (last year: State University of New York at Buffalo)
(7) Massachusetts Institute of Technology (last year: Carleton University)
(8) University of Cambridge (last year: GlaxoSmithKline)
(9) University of Melbourne (last year: University of California Santa Barbara)
(10) University of California Irvine (last year: University of California Irvine)
-----
Top 10 countries that visit the blog:
(1) United States (18,651 visits)
(2) United Kingdom (5,076 visits)
(3) Germany (3,109 visits)
(4) Canada (2,648 visits)
(5) Australia (2,588 visits)
(6) Denmark (2,414 visits)
(7) India (1,856 visits)
(8) Japan (1,081 visits)
(9) Sweden (932 visits)
(10) New Zealand (926 visits)
-----
Top 10 countries that visit the blog:
(1) United States (18,651 visits)
(2) United Kingdom (5,076 visits)
(3) Germany (3,109 visits)
(4) Canada (2,648 visits)
(5) Australia (2,588 visits)
(6) Denmark (2,414 visits)
(7) India (1,856 visits)
(8) Japan (1,081 visits)
(9) Sweden (932 visits)
(10) New Zealand (926 visits)
Tuesday, October 06, 2009
How to Turn an Amine Into a Leaving Group
Leaving group activation of alcohols followed by nucleophilic substitution is routine stuff for the synthetic organic chemist. Just make the tosylate, nosylate, mesylate, triflate.... and thin gs generally go according to plan. However, what if you are stuck with an amine and want to substitute it with a nucleophile. There are a number of ways to do this but it's not just a walk in the park. Until recently I had never had to do this but then one fine morning I wanted to do the reaction above. How does one go about doing t
gs generally go according to plan. However, what if you are stuck with an amine and want to substitute it with a nucleophile. There are a number of ways to do this but it's not just a walk in the park. Until recently I had never had to do this but then one fine morning I wanted to do the reaction above. How does one go about doing t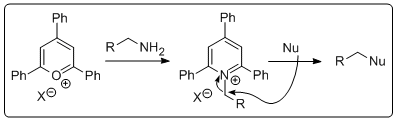 his? Is there a simple method by which I could activate the amine and displace it with the anion of 2-nitropropane, followed by a simple reduction to get the amine I wanted? Well, as it turns out Katritzky and co-workers published a paper in 1979 introducing triphenylpyrylium salts that can convert amines to leaving groups. Granted, the atom economy in this process is (to say th
his? Is there a simple method by which I could activate the amine and displace it with the anion of 2-nitropropane, followed by a simple reduction to get the amine I wanted? Well, as it turns out Katritzky and co-workers published a paper in 1979 introducing triphenylpyrylium salts that can convert amines to leaving groups. Granted, the atom economy in this process is (to say th e least) poor. However, the required pyrylium salt is commertcially available at a resonable price. All you do is stir it up with the amine. Prior to adding the amine the suspension is pale yellow and then when you toss the amine in it becomes a deep red slurry. In the photo the amine has just been added. It's always exciting with a bit of colour if your an organic chemist. The product is isolated by filtration. In this case the pyridinium salt was isolated as a light brown solid in 63% yield, perfectly clean by NMR. Next the pyridinium salt was treated with deprotonated 2-nitropropane in hot DMSO to give the nitro compound that was reduced using old school conditions. Interestingly, we could not get any reduction AT ALL of the nitro compound by catalytic hydrogentaion (at atmospheric pressure). Very odd! I would have ex
e least) poor. However, the required pyrylium salt is commertcially available at a resonable price. All you do is stir it up with the amine. Prior to adding the amine the suspension is pale yellow and then when you toss the amine in it becomes a deep red slurry. In the photo the amine has just been added. It's always exciting with a bit of colour if your an organic chemist. The product is isolated by filtration. In this case the pyridinium salt was isolated as a light brown solid in 63% yield, perfectly clean by NMR. Next the pyridinium salt was treated with deprotonated 2-nitropropane in hot DMSO to give the nitro compound that was reduced using old school conditions. Interestingly, we could not get any reduction AT ALL of the nitro compound by catalytic hydrogentaion (at atmospheric pressure). Very odd! I would have ex pected to see at least a few percent of the reduced stuff. Any ideas out there? Anyway, the amine was isolated in good yield over two steps after a short (2 cm tall) DCVC column. Yes a wastefull method but it is simple and fast. D!
pected to see at least a few percent of the reduced stuff. Any ideas out there? Anyway, the amine was isolated in good yield over two steps after a short (2 cm tall) DCVC column. Yes a wastefull method but it is simple and fast. D!
 gs generally go according to plan. However, what if you are stuck with an amine and want to substitute it with a nucleophile. There are a number of ways to do this but it's not just a walk in the park. Until recently I had never had to do this but then one fine morning I wanted to do the reaction above. How does one go about doing t
gs generally go according to plan. However, what if you are stuck with an amine and want to substitute it with a nucleophile. There are a number of ways to do this but it's not just a walk in the park. Until recently I had never had to do this but then one fine morning I wanted to do the reaction above. How does one go about doing t his? Is there a simple method by which I could activate the amine and displace it with the anion of 2-nitropropane, followed by a simple reduction to get the amine I wanted? Well, as it turns out Katritzky and co-workers published a paper in 1979 introducing triphenylpyrylium salts that can convert amines to leaving groups. Granted, the atom economy in this process is (to say th
his? Is there a simple method by which I could activate the amine and displace it with the anion of 2-nitropropane, followed by a simple reduction to get the amine I wanted? Well, as it turns out Katritzky and co-workers published a paper in 1979 introducing triphenylpyrylium salts that can convert amines to leaving groups. Granted, the atom economy in this process is (to say th e least) poor. However, the required pyrylium salt is commertcially available at a resonable price. All you do is stir it up with the amine. Prior to adding the amine the suspension is pale yellow and then when you toss the amine in it becomes a deep red slurry. In the photo the amine has just been added. It's always exciting with a bit of colour if your an organic chemist. The product is isolated by filtration. In this case the pyridinium salt was isolated as a light brown solid in 63% yield, perfectly clean by NMR. Next the pyridinium salt was treated with deprotonated 2-nitropropane in hot DMSO to give the nitro compound that was reduced using old school conditions. Interestingly, we could not get any reduction AT ALL of the nitro compound by catalytic hydrogentaion (at atmospheric pressure). Very odd! I would have ex
e least) poor. However, the required pyrylium salt is commertcially available at a resonable price. All you do is stir it up with the amine. Prior to adding the amine the suspension is pale yellow and then when you toss the amine in it becomes a deep red slurry. In the photo the amine has just been added. It's always exciting with a bit of colour if your an organic chemist. The product is isolated by filtration. In this case the pyridinium salt was isolated as a light brown solid in 63% yield, perfectly clean by NMR. Next the pyridinium salt was treated with deprotonated 2-nitropropane in hot DMSO to give the nitro compound that was reduced using old school conditions. Interestingly, we could not get any reduction AT ALL of the nitro compound by catalytic hydrogentaion (at atmospheric pressure). Very odd! I would have ex pected to see at least a few percent of the reduced stuff. Any ideas out there? Anyway, the amine was isolated in good yield over two steps after a short (2 cm tall) DCVC column. Yes a wastefull method but it is simple and fast. D!
pected to see at least a few percent of the reduced stuff. Any ideas out there? Anyway, the amine was isolated in good yield over two steps after a short (2 cm tall) DCVC column. Yes a wastefull method but it is simple and fast. D!Saturday, October 03, 2009
Stereogenofobia and Electra's Art
 I have previosuly been moaning about the fear of stereogenic centers that industry seems to suffer from here. So it was with great pleasure that I read Derek Lowe's latest contribution to Chemistry World were he appears to share some of my views.
I have previosuly been moaning about the fear of stereogenic centers that industry seems to suffer from here. So it was with great pleasure that I read Derek Lowe's latest contribution to Chemistry World were he appears to share some of my views.Derek Lowe is a medicinal chemist running the hugely succesfull blog In the Pipeline. If you don't already frequent this blog you should get started. He shares interesting stuff about all aspects from synthetic organic chemistry, science and society, what's going on in industry etc.
---
On a completely different note, I have added a new link to the Coffee Break section (Bottom right of the page) called Electra Lady Land. A friend of mine is an artist and I am a great fan of her work so I have decided to promovate her stuff here. Go have a look at her paintings. D!
---
On a completely different note, I have added a new link to the Coffee Break section (Bottom right of the page) called Electra Lady Land. A friend of mine is an artist and I am a great fan of her work so I have decided to promovate her stuff here. Go have a look at her paintings. D!
Thursday, October 01, 2009
Making the cut
 I keep thinking that I'm all done paper pushing and then somehow magically I'm back at it full force. So recently I've submitted four grant proposals, written a chapter for a book, started teaching again and in parallel I'm doing a half hearted attempt at some lab work. So the last thing I needed to see was this paper Don't read that paper if you are a suicidal post doc.
I keep thinking that I'm all done paper pushing and then somehow magically I'm back at it full force. So recently I've submitted four grant proposals, written a chapter for a book, started teaching again and in parallel I'm doing a half hearted attempt at some lab work. So the last thing I needed to see was this paper Don't read that paper if you are a suicidal post doc.I guess it's the life I've picked but it sure is tempting to bail out and get a "real job" as my mum calls it. Anyway, posting is about to resume with some exciting stuff on how to turn a primary amine into a leaving group. Sigh, D
Subscribe to:
Comments (Atom)


 orcid.org/0000-0003-3926-7047
orcid.org/0000-0003-3926-7047


